High Blood Sugar
Anxiety
Gluten Sensitivity
Gut Inflammation
Blood Pressure
IBS
Mood
Insomnia
PTSD
Mood Swings
Overweight
Memory Performance
Sexual Dysfunction
PCOS
Psoriasis
Joint Pain
Attention/ADHD
Chronic Fatigue / Tiredness
Allergies
Asthma
Acne
Tinnitus
Eczema
Food Allergy
Vitamin B6
Vitamin E
Restless Leg Syndrome
Grinding Teeth
Vitamin A
Magnesium
Zinc
Heart Health
Migraines
(High) Cholesterol
Headache
Chronic Pain
Back pain
Shoulder & Neck Pain
Stress
Inflammation
Omega-3 needs
Salt Sensitivity
Endurance
Power performance
Strength
Exercise recovery
Brain Fog
Female Fertility
Longevity
Addiction
Erectile Dysfunction
Male Infertility
MTHFR
Joint Inflammation
GERD
Ulcers
Sleep Apnea
Periodontitis
Varicose Veins
H. pylori
Liver Health
Canker Sores
Gallstones
Kidney Health
Gout
Hair Loss (Male-Pattern Baldness)
Riboflavin
Urticaria
Rosacea
Carpal Tunnel Syndrome
Sinus Congestion
Cavities
Artery Hardening
Vertigo
Vitiligo
Myopia
Indigestion
Excessive Sweating
Testosterone – Males
Yeast infection (Candida)
Endometriosis
Tobacco addiction
Alcohol addiction
Uterine fibroids
Length of menstrual cycle
UTI
OCD
Kidney Stones
Vitamin B12
Vitamin C
Vitamin D
Folate
Iron
Eating Disorders
Bone Health
Hypothyroidism
Hyperthyroidism
Sugar Cravings
Hearing/difficulty problem /Hearing loss
Painful Periods
Palpitations
Hemorrhoids
Hypotension
Bladder Control
Constipation
Appendicitis
Low Blood Sugar
Irregular Periods
Metabolic rate
Visceral fat
Lung Health
Anemia
Calcium
Cognition
Cognitive Decline
Seasonal Low Mood
Vitamin K
Phosphate
HRV
Cluster headaches
Knee Pain
Hip Pain
Selenium
Low back injury
Dyslexia
Cannabis addiction
Histamine Intolerance
Carnitine
Pesticide Sensitivity
Organophosphate Sensitivity
Cadmium
Lead
Melatonin
FSH
T4
T3
High PTH
Potassium
Coenzyme Q10 (CoQ10)
Chromium
Oxalate Sensitivity
Salicylate Sensitivity
Facial Wrinkles
Age Spots
Ligament Rupture (ACL Injury)
Tendon Injury (Tendinopathy)
Omega 6
Omega 6:Omega 3 Ratio
Arachidonic Acid
Oleic Acid
Alpha-Linolenic Acid
EPA
GLA
Linoleic Acid
DHA
Insulin Resistance
Sperm Motility
Homocysteine
C difficile
Pneumonia
EBV Infection
Gastrointestinal Infection
Chronic Bronchitis
Copper
Skin Elasticity
Skin Hydration
Egg allergy
ApoB
GGT
TIBC
Bioavailable Testosterone (Male)
MPV
Chloride
Free T4
Processing Speed
Short-term memory
TMAO
Air pollution sensitivity
Heart Rate
VO2 Max
Flu
Hair graying
Caffeine-Related Sleep Problems
Groin Hernia
Stretch marks
Droopy Eyelids
Strep infection
Dry eyes
Carbohydrate Consumption
Peanut allergy
Heart rate recovery
Muscle recovery
Jaw Disorders
HPV Infection
Acute Bronchitis
Chlamydia
Genital Herpes
Pancreas inflammation
Executive Function
Pyroglutamic acid
Raynaud’s
Liver Scarring
Dandruff
Bioavailable Testosterone (Female)
Shrimp allergy
Haptoglobin
Milk allergy
Beta-Alanine
Taurine
LDL Particle Size
Diarrhea
Snoring
Uric acid
Phenylalanine
Leucine
Glutamine
Valine
Glycine
Alanine
Lysine
Arginine
Histidine
Tyrosine
Cortisol
DHEAS
Insulin
Prolactin
TSH
Lactate
Ketone Bodies
IL-17A (Th17 Dominance)
Creatine Kinase
Neutrophils
Basophils
Eosinophils
Ferritin
ALT
AST
MCV
Hematocrit
RDW
SHBG
Total Protein
Albumin
MCH
Sodium
MCHC
Alkaline Phosphatase
Monocytes
Ghrelin
IL10 (Th2)
IL-6 (Th2 and Th17)
Iodine
Chili Pepper sensitivity
COMT
DRD2 (Dopamine)
Lectin Sensitivity
Thiamine
Biotin
Mold Sensitivity (Foodborne)
Chronic Lyme
BDNF
Glyphosate sensitivity
BPA Sensitivity
Pregnenolone
Luteinizing Hormone (LH)
Growth Hormone
IgA
Molybdenum
Sensitivity to Dairy (IgG Casein)
Telomere Length
Serotonin (5HIAA)
Non-Celiac Gluten Sensitivity (IgG Gliadin)
Manganese
Klotho
Mold Sensitivity (Airborne)
Amylase
Lipase
Low Sperm Count
Tryptophan
Methionine
Glutamate
Proline
Blood Calcium
Hypertriglyceridemia
HDL Cholesterol
HbA1c
Hemoglobin
Total Cholesterol
LDL Cholesterol
IGF1
Fasting Glucose
Bilirubin (total)
White blood cell count
Red blood cell count
Platelets
eGFR
Creatinine
Estradiol
Neuroticism
Sleep Quality
Lactose Intolerance
Saturated fat
Optimal diet
Unsaturated fat
Achilles tendon injury
Deep sleep
Fat
Response to Stress
Leadership
Ankle injury
Creativity
Hoarding
Protein
Optimal Exercise
Knee Injury
Rotator cuff injury
Extraversion
Risk-Taking
Happiness
Daytime Sleepiness
Morningness
Time spent watching TV
Disliking cilantro
Alcohol Sensitivity
Response to Caffeine
Snacking
Weight Regain
Sleep movement
Wearing glasses or contacts
Educational Attainment
Bitter Taste Sensitivity
Agreeableness
Aggression
Conscientiousness
Openness to experience
Physical activity
Caffeine-Related Anxiety
Naps
SelfDecode uses the only scientifically validated genetic prediction technology for consumers. Read more
The Role of One Receptor in Ulcerative Colitis (FCGR2A)
The FCGR2A gene encodes a receptor that activates the antimicrobial response. One variant in this gene is associated with ulcerative colitis—read on to learn the details and get tailored tips.
FCGR2A in Immunity and Inflammation
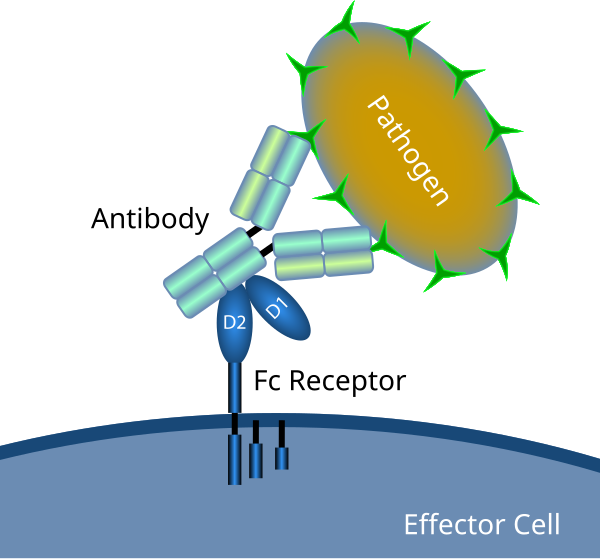
By Fc_receptor_schematic_big.png: en:User: Ciar from w: enderivative work: Rehua (talk)
The FCGR2A gene encodes a specific type of Fc receptor: FcγRIIA or CD32. Immune cells such as monocytes and macrophages express Fc receptors, which recognize antibodies attached to pathogens or infected cells [R].
Fcγ(gamma) receptors recognize IgG antibodies. While they all bind IgG1 and IgG3 antibodies, only FcγRIIA can effectively bind IgG2. Once activated, this receptor sets off different mechanisms that promote inflammation and pathogen removal [R, R, R].
Over-active CD32 can contribute to autoimmunity, while its suppression leads to poor antimicrobial response [R].
FCGR2A encodes a receptor that binds IgG antibodies and signals the immune response against pathogens. An over-active receptor can contribute to inflammation and autoimmunity.
The Role in Ulcerative Colitis
Ulcerative colitis (UC) and Crohn’s disease (CD) are the most frequent subtypes of inflammatory bowel disease (IBD). The cause of IBD lies in a complex interplay of genetic and environmental factors [R].
Ulcerative colitis typically affects the colon and rectum, and involves inflammation of the surface (mucosal) layer [R, R].
Although IBD is considered a T cell-mediated condition, recent evidence implies a dysfunction in B cells and FcγR signaling in IBD development. In patients with IBD, and especially ulcerative colitis, IgG antibodies can flag healthy gut components and trigger an autoimmune reaction [R, R, R].
Ulcerative colitis is a form of autoimmune colon inflammation. Self-destructive IgG antibodies can play a role in this condition via impaired FcγR signaling.
FCGR2A Variant and Ulcerative Colitis Susceptibility
Robust evidence suggests a link between one FCGR2A variant and ulcerative colitis. In a meta-analysis of six trials and over 26,000 participants, the “A” allele at rs1801274 was associated with 21% higher UC rates. All subjects were of European ancestry [R].
A study of over 41,000 European subjects confirmed the association between this variant and IBD. However, some studies haven’t found a significant link with Crohn’s disease, suggesting its primary role in ulcerative colitis [R, R].
The same SNP is associated with ulcerative colitis in Korean and Japanese populations, too [R, R].
The “A” allele at rs1801274 is associated with higher rates of ulcerative colitis across different populations.
IBD Complications and Dietary Factors
A meta-analysis that included 2,287 IBD patients associated this variant with 65% higher chances of IBD-associated colorectal cancer. However, when corrected for other contributing factors, the effect became non-significant [R].
A study of over 165,000 women identified one dietary factor that greatly impacts the link between rs1801274 and ulcerative colitis. In women with the “AA” genotype, iron from animal sources was associated with higher UC rates (3-fold increase for each 1 g of added iron) [R].
On the other hand, iron from animal sources was protective in women with the “GG” genotype [R].
People with rs1801274-A may be more prone to IBD-associated colon cancer. Iron from animal sources could amplify the effects of this variant.
How It Works
The G>A switch at rs1801274 changes one amino acid in the FcγRIIA structure. The “A” allele codes for histidine, which increases the receptor affinity for IgG antibodies, especially IgG2. This change improves pathogen removal but also contributes to autoimmune conditions such as ulcerative colitis [R, R].
The activation of FcγRIIA receptors on the gut immune cells triggers IL-1b production, which in turn drives Th17 immunity and inflammation [R, R].
Other Conditions
This variant has a well-known role in autoimmune conditions, such as:
Changes in the FcγRIIA structure and function can also affect the immune response to HIV, bacterial infections, lymphomas, and more [R, R, R].
Your FCGR2A Results for Ulcerative Colitis
SNP Table
| variant | genotype | frequency | risk allele |
| rs1801274 |
SNP Summary
Primary SNP:
FCGR2A rs1801274
- ‘G’ = not associated with ulcerative colitis
- ‘A’ = associated with higher rates of ulcerative colitis and complications
Population Frequency: Around 47% of European descendants carry one copy, and 26% carry both copies of the “A” allele. This allele is even more common in East Asian populations, where 43% of people have one and 51% have both copies.
Recommendations
Lifestyle
Avoid Stress
Clinical and animal studies have shown the potential of psychological stress to raise IgG antibodies, especially IgG2. Stress can also mimic your gene variant by increasing IL-1b levels and activating Th17 cells [R, R, R, R].
Psychological stress has well-known detrimental effects on gut health and inflammation. It raises gut permeability and the levels of different inflammatory cytokines [R, R].
Many ulcerative colitis patients develop anxiety and depression associated with their debilitating digestive symptoms. In turn, stress may worsen the symptoms of UC. Because of this potential feedback loop, many doctors emphasize the importance of managing the mental health of UC patients [R, R, R, R].
Psychological stress can worsen the impact of your gene variant and play a significant role in ulcerative colitis development. Try to avoid stress and practice relaxation techniques.
Moderate Sun Exposure
Sunlight is the best natural source of vitamin D, which helps combat Th17-associated inflammation. In a trial of 1,470 older people, vitamin D deficiency was associated with higher levels of IgG2 [R, R].
Additionally, UV light may counteract your FCGR2A variant by suppressing Th17 cells [R, R].
Calcium and vitamin D deficiencies are common in ulcerative colitis patients, leading to osteoporosis and joint disorders. The lack of vitamin D is also associated with increased UC severity and duration. Moderate sun exposure will help you get enough of this crucial nutrient [R, R, R].
Moderate sun exposure may lessen the impact of your variant. It supplies vitamin D, which is crucial for ulcerative colitis patients.
Diet
IgG-Based Elimination Diet
Unlike acute allergic reactions, which are IgE-mediated, most delayed food reactions (sensitivities) are IgG-mediated. Based on this feature, doctors have developed special elimination diets, excluding foods that raise IgG levels in sensitive patients [R, R, R].
In a study of 97 ulcerative colitis patients, an IgG-based elimination diet significantly reduced the symptoms and improved the quality of life. This approach may be particularly useful for people with the discussed SNP, given its effect on IgG activity [R].
A special elimination diet can remove the foods that increase IgG levels and thus potentially improve ulcerative colitis.
Limit the Intake of Iron From Animal Sources
As mentioned, iron from animal sources can amplify the impact of rs1801274-A on ulcerative colitis [R].
Red meat is the most concentrated animal source of iron. Increased intake of red or processed meat is associated with higher ulcerative colitis relapses (re-activation) [R, R].
Consider limiting the intake of pork, lamb, and beef. You can replace them with fish, chicken, and plant sources of iron. Of course, measure your ferritin and iron levels to make sure you are still getting enough iron.
To reduce the impact of your variant, limit the intake of red meat and replace it with fish, chicken, and plant sources of iron.
Supplements
Boswellia
Boswellia resin or frankincense is a natural remedy with powerful anti-inflammatory properties. It may counteract the FcγRIIA receptors by reducing IL-1b levels and Th17 activity [R, R, R].
Boswellia extract also has antioxidant activity and protects the intestinal barrier from inflammatory damage [R].
It was effective in the treatment of 30 patients with chronic colitis with minimal side effects. In another trial, Boswellia resin improved ulcerative colitis with 80-82% remission [R, R].
Boswellia resin or frankincense is a powerful anti-inflammatory. It may counteract the FcγRIIA receptors and improve the symptoms of ulcerative colitis.
Curcumin
Curcumin is the active principle of turmeric. It can suppress Th17 cells and lower IL-1b levels [R, R, R].
Multiple clinical trials have produced promising results for curcumin in IBD, and further research is currently underway [R, R, R].
Vitamin D
As mentioned, vitamin D deficiency is associated with higher Th17 activity and IgG levels [R, R].
If you can’t get enough vitamin D from sun exposure, consider taking a supplement. In a meta-analysis of 18 trials and 908 patients, vitamin D supplementation corrected a deficiency and helped prevent IBD relapse [R].
Curcumin and vitamin D may reduce the impact of your FCGR2A variant and help with ulcerative colitis.

Aleksa received his MS in Pharmacy from the University of Belgrade, his master thesis focusing on protein sources in plant-based diets.
Aleksa is passionate about herbal pharmacy, nutrition, and functional medicine. He found a way to merge his two biggest passions—writing and health—and use them for noble purposes. His mission is to bridge the gap between science and everyday life, helping readers improve their health and feel better.
Disclaimer
The information on this website has not been evaluated by the Food & Drug Administration or any other official medical body. This information is presented for educational purposes only, and may not be used to diagnose or treat any illness or disease.
Also keep in mind that the “Risk Score” presented in this post is based only on a select number of SNPs, and therefore only represents a small portion of your total risk as an individual. Furthermore, these analyses are based primarily on associational studies, which do not necessarily imply causation. Finally, many other (non-genetic) factors can also play a significant role in the development of a disease or health condition — therefore, carrying any of the risk-associated genotypes discussed in this post does not necessarily mean you are at increased risk of developing a major health condition.
Always consult your doctor before acting on any information or recommendations discussed in this post — especially if you are pregnant, nursing, taking medication, or have been officially diagnosed with a medical condition.
More gut health blogs
Unlock Personalized Results And So Much More!
Shipping Worldwide
30-Days Money-Back Guarantee*
US & EU Based Labs & Shipping
Essential Bundle
SelfDecode DNA Kit Included
- 24/7 AI Health Coach
- 1500+ Comprehensive DNA Health Reports
- Personalized Diet, Supplement, & Lifestyle Recommendations
- Lifestyle Risk Assessments
- Unlimited access to Labs Analyzer
HSA & FSA Eligible
HSA & FSA Eligible
Essential
Bundle
SelfDecode DNA Kit Included
- Everything in essential
- Detox Pathways
- Methylation Pathway
- Histamine Pathway
- Dopamine & Norepinephrine Pathway
- Serotonin & Melatonin Pathway
- +130 Medical Reports
- 25+ Longevity Screener Risk Assessments
- Odds ratios to evaluate your risk for 25+ medical conditions
- 10-year risk scores to prioritize health conditions
- Lifetime risk scores to plan for long-term health
HSA & FSA Eligible
Ultimate Bundle
SelfDecode DNA Kit Included
- Everything in essential+
- Medication Check (PGx testing) for 50+ medications
- DNAmind PGx Report
- 40+ Family Planning (Carrier Status) Reports
- Ancestry Composition
- Deep Ancestry (Mitochondrial)
Limited Time Offer 25% Off
Not ready for a full bundle?
Individual DNA Reports
- HSA & FSA Eligible

* SelfDecode DNA kits are non-refundable. If you choose to cancel your plan within 30 days you will not be refunded the cost of the kit.
We will never share your data
We follow HIPAA and GDPR policies
We have World-Class Encryption & Security
Rated 4.7/5 from 750+ reviews
200,000+ users, 2,000+ doctors & 100+ businesses
SelfDecode is a personalized health report service, which enables users to obtain detailed information and reports based on their genome. SelfDecode strongly encourages those who use our service to consult and work with an experienced healthcare provider as our services are not to replace the relationship with a licensed doctor or regular medical screenings.